
"Program Developed for CO 2 System Calculations". Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters (3rd ed.). C T is a key parameter when making measurements related to the pH of natural aqueous systems, and carbon dioxide flux estimates.Ĭ T = + + : CS1 maint: location missing publisher ( link) It is customary to express carbon dioxide and carbonic acid simultaneously as CO 2*. The inorganic carbon species include carbon dioxide, carbonic acid, bicarbonate anion, and carbonate. The processes associated with each arrow describe the transformation associated with the transfer of carbon from one reservoir to another. Inorganic carbon species Carbon is separated into four distinct pools based on whether it is organic or inorganic, and whether it is dissolved or particulate. The programs are widely used by oceanographers and limnologists to understand and predict chemical equilibria in natural waters. Their core function is to use any two of the four central inorganic carbon system parameters ( pH, alkalinity, dissolved inorganic carbon, and partial pressure of carbon dioxide) to calculate various chemical properties of the system.
#CHARGE OF CARBON IN CO3 SOFTWARE#
This software calculate chemical equilibria for aquatic inorganic carbon species and parameters. Since 1998, a family of software programs called CO2SYS has been widely used. įor most of the 20th century, chemical equilibria in marine and freshwater systems was calculated according to various conventions, which led to discrepancies among laboratories' calculations and limited scientific reproducibility. Given any two of the four central inorganic carbon system parameters (pH, alkalinity, dissolved inorganic carbon, partial pressure of carbon dioxide) the remainder may be derived by solving a system of equations that adhere to the principles of chemical thermodynamics. Variables like alkalinity and dissolved (or total) inorganic carbon further define a mass and charge balance that constrains the total state of the system.

The relative amounts of each species in a body of water depends on physical variables including temperature and salinity, as well as chemical variables like pH and gas partial pressure. These species include dissolved carbon dioxide, carbonic acid, bicarbonate anion, carbonate anion, calcium carbonate, magnesium carbonate, and others. The aquatic inorganic carbon system is composed of the various ionic, dissolved, solid, and/or gaseous forms of carbon dioxide in water. Inorganic carbon is found primarily in simple compounds such as carbon dioxide ( CO 2), carbonic acid ( H 2CO 3), bicarbonate ( HCO − 3), and carbonate ( CO 2− 3).
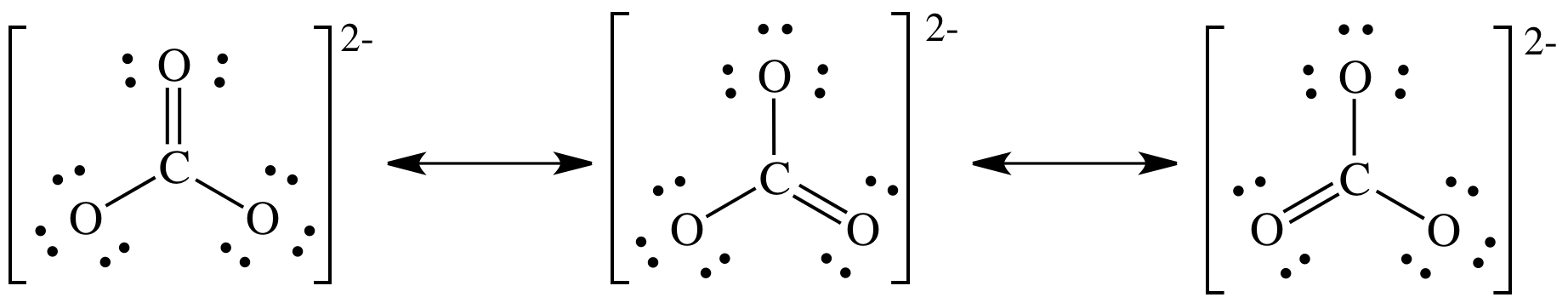
Organic carbon forms the backbone of key components of organic compounds such as proteins, lipids, carbohydrates, and nucleic acids. Total inorganic carbon ( C T or TIC) is the sum of the inorganic carbon species.Ĭarbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition.


 0 kommentar(er)
0 kommentar(er)
